Part 2: What Can Be And Is Being Done About It

Part 1 explains how Alzheimer’s begins, and just as importantly, when. The earliest changes happen long before memory fades — in how the brain uses energy, manages inflammation, and maintains its structural integrity. These shifts develop gradually and follow recognisable patterns, which means there is time to intervene with purpose.
Part 2 explores five areas where that influence appears strongest: sleep, movement, nutrition, cholesterol, and metabolic health. It ends with a look at emerging treatments designed to ease symptoms while also addressing the disease process itself.
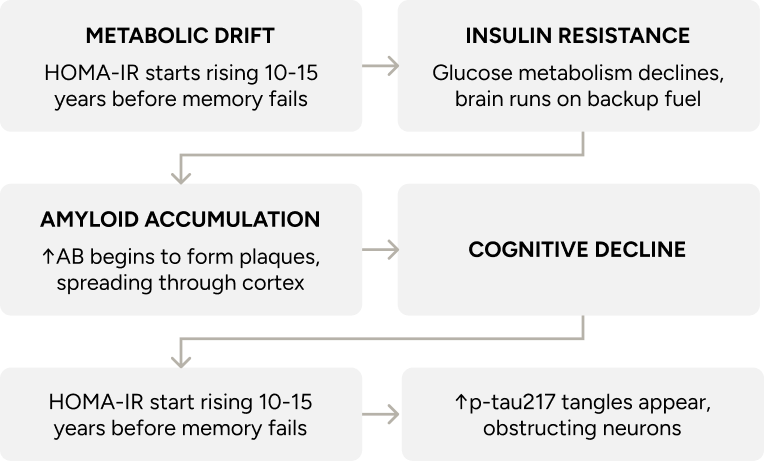
Cognitive decline occurs gradually, often preceded by shifts in brain metabolism, inflammation, and neural connections. These early changes tend to follow patterns that respond to input. In systems biology, small forces applied consistently can alter long-term direction⁴⁴ ⁴⁵ ⁴⁶ ⁴⁷.
The following 5 levers, pulled early, appear to reduce risk and preserve function⁴⁶.

Sleep is an active, restorative process during which the brain clears waste, consolidates memories, and resets defences. Deep sleep, especially slow-wave sleep, activates the glymphatic system (a clearance pathway that flushes out neurotoxic proteins like beta-amyloid)⁴⁸.
Sleep to support long-term cognitive function needs to be:

Multiple factors shape sleep architecture. Tracking tools now reveal patterns in efficiency⁵³, fragmentation⁵⁴, and sleep stages⁵⁵. Evening light, caffeine, or alcohol disrupt timing by delaying melatonin secretion and suppressing deep sleep.⁵⁶

Physical training supports the growth of new neurons, enhances mitochondrial function, and improves metabolic health.⁵⁷ Regular exercise has been linked to significantly lower AD risk and improved cognitive performance across the lifespan.⁵⁸
What to train to reduce AD risk:

Specific dietary patterns and nutrients can reduce neuroinflammation, improve synaptic plasticity and metabolic pathways, impacting cognitive health and AD risk⁶⁵. In particular, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) showed that combining diet, exercise, cognitive training, and vascular monitoring can maintain or improve cognition in older adults at risk of decline⁶⁶.
Specific nutritional interventions that may reduce AD risk:

Advanced lipid profiling reveals patterns linked to cognitive decline—even in individuals with “normal” total cholesterol⁷³. Smaller, denser LDL particles are more likely to oxidise and breach the blood–brain barrier. Mendelian randomisation (MR) studies confirm that elevated triglycerides are causally linked to cognitive impairment, likely through insulin resistance mechanisms and compromised vascular health⁷⁴ ⁷⁵.
Similarly, higher HDL cholesterol levels appear protective against cognitive decline according to genetic evidence. High lipoprotein(a), a genetically determined LDL variant, is associated with increased risk of stroke and white matter lesions. However, MR studies have not established a direct causal role in Alzheimer’s disease⁷⁶.
As a result, lipid targets now converge across cardiovascular and neurological domains⁷⁷. Thresholds associated with long-term brain health include LDL below 1.8 mmol/L,⁷⁸ HDL above 1.6 mmol/L,⁷⁹ triglycerides under 1.1 mmol/dL,⁸⁰ and Lp(a) below 75 nmol/L⁸¹ — values that define a lipid environment optimised for synaptic stability, vascular integrity, and cognitive resilience⁸².

The most sensitive markers of early metabolic change linked to cognitive decline are fasting insulin, HbA1c, and the glucose tolerance test (which is why I test these in every patient at regular intervals). They reflect how efficiently the body processes glucose at rest. Even modest elevations in these values have been linked to reduced cerebral glucose metabolism, increased amyloid burden, and impaired executive function⁸³ ⁸⁴.
Values associated with metabolic stability — and by extension, cognitive protection — include HbA1c below 5.5%, fasting insulin under 5 µIU/mL, and HOMA-IR below 1.5. Continuous glucose monitoring captures postprandial spikes and daily variability that static labs often miss, offering a more dynamic view of glucose regulation under real-world conditions. Lipoprotein profiling adds further resolution, linking metabolic flexibility with long-term structural and cognitive integrity⁸⁵.
Figure 3. Five Levers for Alzheimer’s Prevention
If you’d asked me ten years ago what we had for Alzheimer’s, I would’ve said donepezil, and that’s it. It offered symptomatic relief, particularly in early stages, but did not affect the disease’s progression or underlying pathology⁸⁶. Now, the field is gaining traction with interventions that match the complexity of the disease.
Today, AD is a field of entrance research, and there is optimism that a cure or at very least an effective treatment will be available within 10 years. Here are some of the approaches scientists and clinicians are taking:
ApoE4-Targeted Approaches
Several new approaches specifically target ApoE4 biology, either by reducing its production, modifying its genetic code, or counteracting its downstream effects. The table below outlines leading interventions currently under investigation:
Table 2. Emerging Alzheimer’s Interventions by Mechanism and Development Stage
Leading Centres of Excellence
The fight against Alzheimer’s now operates a global network of specialised research centres collaborating across disciplines. Breakthroughs in one area rapidly inform progress in others. From molecular mechanisms to clinical applications, this model accelerates discoveries beyond what any single centre could achieve.
These centres collaborate through networks like the Alzheimer’s Disease Research Centers (ADRCs) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI), creating platforms where data sharing accelerates discovery across the entire field.
Alzheimer’s disease represents a convergence of genetic predisposition, metabolic dysfunction, and environmental factors that disrupt fundamental mechanisms of brain health long before symptoms arise.
While the presence of the ApoE ε4 allele increases susceptibility, its trajectory is neither deterministic nor irreversible. Early intervention targeting metabolic stability, lipid management, sleep optimisation, and neuroinflammation offers the best defence against cognitive decline.
As our diagnostic capabilities expand and targeted therapies evolve, the paradigm shifts from passive observation to proactive prevention. Clinicians need to lead this shift by giving patients the tools and knowledge to protect their brain health for life.
From Drift to Dementia